 |
What is Cathodic protection? |
Cathodic protection (CB) is the supply of direct current to steel objects that are in the ground or water. It is a way of protecting steel objects from corrosion. The potential is lowered by the current on the steel pipes. This lowered potential slows down the corrosion process.
How does cathodic protection work?
Cathodic protection works to prevent oxidation, stopping corrosion. When metals oxidize, metal atoms enter into a chemical bond with oxygen. Electrons flow from a place of high electrical potential to a place of low potential. Cathodic protection eliminates this difference in potential. The electric current cannot flow and the oxidation process cannot take place. This is how we prevent corrosion.
Cathodic protection is usually applied as a secondary method of protection against corrosion. The primary protection against corrosion is usually obtained by means of a coating. This coating reduces the protective current required for cathodic protection. Cathodic protection on a coated object has the additional advantage, that in places where the coating is damaged or other places with reduced (electrical) resistance, the current density and therefore the protection increases locally.
Two forms of cathodic protection
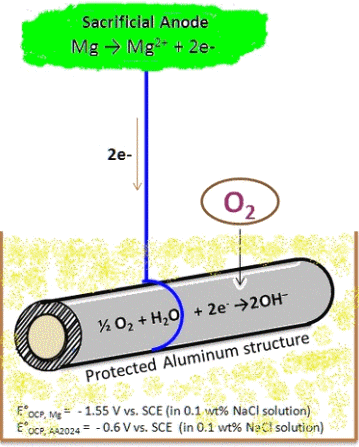
1. With self sacrificial anodes
In this process, a piece of metal, which is base metal, is coupled to the pipe to be protected. This piece of metal forms the anode and is usually made of zinc, aluminum or magnesium. This coupling creates a protective current for the steel object. The anode is sacrificed, as it were, for the protection of the object.
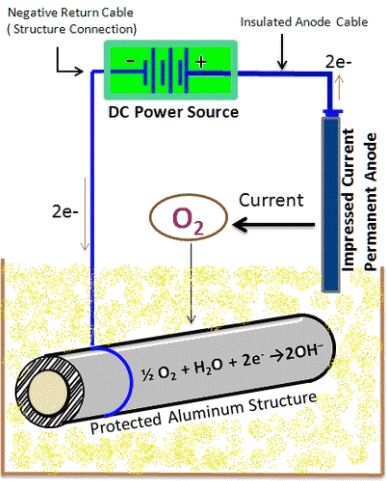
2. Impressed current CP method
A second method is to provide the protective current with an external power Reference(s). This is called an Impressed Current Cathodic Protection system. The external current source is usually provided by a rectifier in combination with a base metal piece. Which anode is best to use depends on several factors and is best determined by a CB specialist.
2 Images above:
University of Southern Mississippi
Michael Blanton
Maintenance of Cathodic Protection
State and federal regulations require that the metallic components that routinely contain petroleum be protected from corrosion. This can include the tank, piping, flex connectors, or any other tank or pipe fitting constructed of metal. These metal components are cathodically protected through cathodic isolation, a galvanic (sacrificial) anode system, or an impressed current system. All types of cathodic protection require routine inspection and maintenance, but some require more than others.
Cathodic isolation requires relatively low maintenance, at least compared to the other methods of protection. Essentially, you must keep the components free from contact with soil, backfill, liquid, and debris. This can be done by removing the corrosive elements or by using an isolation sleeve/boot. In most cases, this issue can be addressed by the owner or operator and is as simple as removing the liquid of soil/backfill so that the metal component is completely clear. These areas must be inspected regularly to ensure that the issue does not reoccur.
CATHODIC TUBE SHEET PROTECTION
 In this picture, the zinc anode is corroded and the tube sheet is protected
In this picture, the zinc anode is corroded and the tube sheet is protectedImage: hareeshkv.blogspot.com