
 |
NITROGEN |
Reference(s)..
THE BRITISH PETROLEUM COMPANY - hazards of nitrogen and catalyst handling booklet TEN

Safe use of Nitrogen (N)
Introduction
The atmosphere we live in, the air we breathe every day consists of 78% Nitrogen, 21% Oxygen and 1% traces of other gases. But only oxygen is vital and essential to human beings for respiration/survival. Without sufficient oxygen, i.e. if oxygen level falls below 16%, we will die of asphyxiation.
Nitrogen gas behaves somewhat like a diluent or buffer gas in the atmosphere. Nitrogen in itself is inert, stable, non-reactive and non-toxic, but too much Nitrogen reduces the oxygen content in the atmosphere, creating an invisible condition that can kill.
If the earth was without Nitrogen but filled with just oxygen, then fires will burn out of control and steel structures will quickly rust away! Therefore, Nitrogen is an effective diluent or buffer gas that we can't live without, yet too much of it will deprive us from the vital oxygen, which can lead to asphyxiation, and even death within seconds.
Nitrogen is widely used for various purposes in refineries and petrochemical plants, for example, to provide an inert atmosphere, to purge a vessel of hydrocarbons, for blanketing and padding storage tanks in order to prevent explosions and fires.
Nitrogen is odorless and colourless. It can kill without giving any warning. Therefore, it is known as the invisible killer that has caused many fatalities in the refineries worldwide.
Nitrogen is one of the most DANGEROUS GASES found in refineries and chemical plants
Properties of Nitrogen
Nitrogen is not toxic since about 78% of the air we breathe contains this gas. The mechanism of Nitrogen gassing is different to that of hydrogen sulphide (H2S). Whilst H2S has a direct toxic effect which is well documented, Nitrogen rich atmospheres will asphyxiate due to a reduction in the oxygen content of the inhaled gases. The typical physiological effects of varying degrees of oxygen deficiency are also well documented..
| Oxygen (%vol) |
Effects and Symptoms |
| 23.5 | Maximum "Safe Level" (23% is often the High level alarm of most O2 detectors) |
| 21 | Typical O2 concentration in air |
| 19.5 | Minimum "Safe Level" (19% is often the Low level alarm of most O2 detectors) |
| 15-19 | First sign of hypoxia. Decreased ability to work strenuously. May induce early symptoms in persons with coronary, pulmonary or circulatory problems |
| 12-14 | Respiration increases with exertion, pulse up, impaired muscular coordination, perception and judgment |
| 10-12 | Respiration further increases in rate and depth, poor judgment, lips blue |
| 8-10 | Mental failure, fainting, unconsciousness, ashen face, blueness of lips, nausea, vomiting, inability to move freely |
| 6-8 | 6 minutes - 50% probability of death, 8 minutes - 100% probability of death |
| 4-6 | Coma in 40 seconds, convulsions, respiration ceases, death |
When a person enters an oxygen-deprived atmosphere, the oxygen level in the arterial blood drops to a low level within 5 to 7 seconds. Loss of consciousness follows in 10-12 seconds and if the person does not receive any oxygen within 2-4 minutes, heart failure and death ensue.
NOTE.. There is also a risk of suffocation with all compressed gases (for example - argon, CO2, helium, etc.), which either replaces the oxygen or consumes it. This risk also exists in situations where there is a large consumption of oxygen (fires, and rusting in ballast tanks of a ship or water tanks, etc.)
Uses and Hazards of Nitrogen
Nitrogen has numerous safety applications in our plants..
As a gas..
• for inerting equipment to prevent flammable atmospheres
• for preparing equipment for maintenance by purging out hydrocarbons
• for removal of air / oxygen in equipment before start up
• for blanketing tanks to prevent the ingress of air
• for specific welding operations
• for "mothballing" equipment to avoid the rusting process
• for use as fire-fighting agent as it removes air
As a liquid..
• for cooling purposes in the laboratory, freezing a pipeline, etc
• for storage and transportation of Nitrogen in large quantities
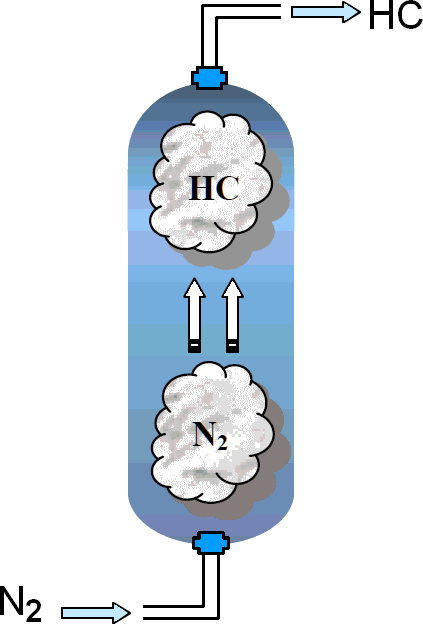
Removal of hydrocarbon vapour prevents possibility of a flammable
atmosphere in preparation for maintenance
What are the Hazards of Nitrogen..
Nitrogen is not toxic since about 78% of the air we breathe contains this gas. However, it is not harmless and it has NO SMELL.
As a gas..
• It can cause suffocation by replacing the oxygen in a confined area
• Its presence will give false readings when using explosimeters or flammable gas detectors
• And, like other compressed gases, there are the risks related to its pressurised containment when it is stored in high pressure cylinders
As a liquid..
• The same as the gas, when it evaporates
• By creating an intense coldness (-196oC) that can cause frostbite, crack steel equipment and explode tyres
• It boils at a colder temperature than oxygen thereby condensing the oxygen in the air (which can then form explosive mixtures with other vapours or cause a violent reaction in contact with organic substances)

A chemical (gas or vapour) that can cause death or unconsciousness by suffocation.
Simple aphyxiants such as Nitrogen, displace oxygen in air.
They become especially dangerous in confined or enclosed spaces.
Chemical asphyxiants, such as carbon monoxide and hydrogen sulfide,
interfere with the body's ability to absorb or transport oxygen to the tissues.
Hazards of Nitrogen Asphyxiation
Related Post(s)

CAN YOU SEE OR SMELL THE INVISIBLE KILLER?...