
 |
Lower and Upper Explosive Limits for Flammable Gases and Vapors |

%LEL / %UEL / PPM / PID
Before a fire or explosion can occur, three conditions must be met simultaneously.
A fuel (ie. combustible gas) and oxygen (air) must exist in certain proportions, along with an ignition Reference(s), such as a spark or flame. The ratio of fuel and oxygen that is required varies with each combustible gas or vapor.
The minimum concentration of a particular combustible gas or vapor necessary to support its combustion in air is defined as the Lower Explosive Limit (LEL) for that gas. Below this level, the mixture is too "lean" to burn. The maximum concentration of a gas or vapor that will burn in air is defined as the Upper Explosive Limit (UEL). Above this level, the mixture is too "rich" to burn. The range between the LEL and UEL is known as the flammable range for that gas or vapor.
Methane - LEL.. 5% by volume in Air / UEL.. 17% by volume in Air
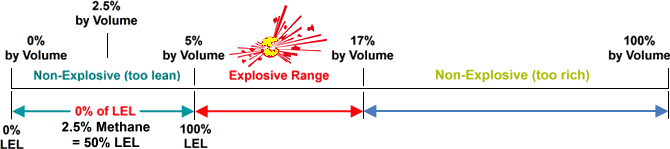
Visual example to show where on the scale % of LEL is measured
Lower and Upper Explosive Limits
The values shown in the table below are valid only for the conditions under which they were determined (usually room temperature and atmospheric pressure using a 2 inch tube with spark ignition). The flammability range of most materials expands as temperature, pressure and container diameter increase. All concentrations in percent by volume.
| Gas | LEL | UEL |
| Acetone | 2.6 | 13 |
| Acetylene | 2.5 | 100 |
| Acrylonitrile | 3 | 17 |
| Allene | 1.5 | 11.5 |
| Ammonia | 15 | 28 |
| Benzene | 1.3 | 7.9 |
| 1.3 Butadiene | 2 | 12 |
| Butane | 1.8 | 8.4 |
| n Butanol | 1.7 | 12 |
| 1 Butene | 1.6 | 10 |
| Cis 2 Butene | 1.7 | 9.7 |
| Trans 2 Butene | 1.7 | 9.7 |
| Butyl Acetate | 1.4 | 8 |
| Carbon Monoxide | 12.5 | 74 |
| Carbonyl Sulfide | 12 | 29 |
| Chlorotrifluoro ethylene | 8.4 | 38.7 |
| Cumene | 0.9 | 6.5 |
| Cyanogen | 6.6 | 32 |
| Cyclohexane | 1.3 | 7.8 |
| Cyclopropane | 2.4 | 10.4 |
| Deuterium | 4.9 | 75 |
| Diborane | 0.8 | 88 |
| Dichlorosilane | 4.1 | 98.8 |
| Diethylbenzene | 0.8 | |
| 1.1 Difluoro 1 Chloroethane | 9 | 14.8 |
| 1.1 Difluoroethane | 5.1 | 17.1 |
| 1.1 Difluoro ethylene | 5.5 | 21.3 |
| Dimethylamine | 2.8 | 14.4 |
| Dimethyl Ether | 3.4 | 27 |
| 2.2 Dimethyl propane | 1.4 | 7.5 |
| Ethane | 3 | 12.4 |
| Ethanol | 3.3 | 19 |
| Ethyl Acetate | 2.2 | 11 |
| Ethyl Benzene | 1 | 6.7 |
| Ethyl Chloride | 3.8 | 15.4 |
| Ethylene | 2.7 | 36 |
| Ethylene Oxide | 3.6 | 100 |
| Gasoline | 1.2 | 7.1 |
| Heptane | 1.1 | 6.7 |
| Hexane | 1.2 | 7.4 |
| Hydrogen | 4 | 75 |
| Hydrogen Cyanide | 5.6 | 40 |
| Hydrogen Sulfide | 4 | 44 |
| Isobutane | 1.8 | 8.4 |
| Isobutylene | 1.8 | 9.6 |
| Isopropanol | 2.2 | |
| Methane | 5 | 17 |
| Methanol | 6.7 | 36 |
| Methylac etylene | 1.7 | 11.7 |
| Methyl Bromide | 10 | 15 |
| 3 Methyl 1 Butene | 1.5 | 9.1 |
| Methyl Cellosolve | 2.5 | 20 |
| Methyl Chloride | 7 | 17.4 |
| Methyl Ethyl Ketone | 1.9 | 10 |
| Methyl Mercaptan | 3.9 | 21.8 |
| Methyl Vinyl Ether | 2.6 | 39 |
| Monoethy lamine | 3.5 | 14 |
| Monomethy lamine | 4.9 | 20.7 |
| Nickel Carbonyl | 2 | |
| Pentane | 1.4 | 7.8 |
| Picoline | 1.4 | |
| Propane | 2.1 | 9.5 |
| Propylene | 2.4 | 11 |
| Propylene Oxide | 2.8 | 37 |
| Styrene | 1.1 | |
| Tetrafluoro ethylene | 4 | 43 |
| Tetrahydrofuran | 2 | |
| Toluene | 1.2 | 7.1 |
| Trichloro ethylene | 12 | 40 |
| Trimethylamine | 2 | 12 |
| Turpentine | 0.7 | |
| Vinyl Acetate | 2.6 | |
| Vinyl Bromide | 9 | 14 |
| Vinyl Chloride | 4 | 22 |
| Vinyl Fluoride | 2.6 | 21.7 |
| Xylene | 1.1 | 6.6 |
Principles of Gas Detection
One of the many requirements for entering confined spaces is the measurement for flammable gases. Prior to entry of a confined space, the level of flammable gases must be below 10% of LEL.
The most common sensor used for measuring LEL is the Wheatstone bridge/catalytic bead/pellistor sensor ("Wheatstone bridge").
LEL Sensors Explained
A Wheatstone bridge LEL sensor is simply a tiny electric stove with two burner elements. One element has a catalyst (such as platinum) and one doesn't. Both elements are heated to a temperature that normally would not support combustion.
However, the element with the catalyst "burns" gas at a low level and heats up relative to the element without the catalyst. The hotter element has more resistance and the Wheatstone bridge measures the difference in resistance between the two elements, which correlates to LEL.

Unfortunately, Wheatstone bridge sensors fail to an unsafe state; when they fail, they indicate safe levels of flammable gases. Failure and/or poisoning of Wheatstone bridge LEL sensor can only be determined through challenging Wheatstone bridge sensors with calibration gas.
LEL Sensors Limitations
Two mechanisms affect the performance of Wheatstone bridge LEL sensors and reduce their effectiveness when applied to all but methane..
- Gases burn with different heat outputs
Some gases burn hot and some burn relatively cool. These differing physical characteristics lead to difficulties when using LEL sensors. For example, 100% of LEL Methane (5% methane by volume) burns with twice the heat of 100% of LEL Propane (2.0 propane by volume).
- Heavier hydrocarbon vapors have difficulty diffusing into LEL sensors and reduce their output
Some Heavier hydrocarbon vapors have difficulty diffusing through the sintered metal flame arrestor on LEL sensors. This flame arrestor is necessary to prevent the sensor itself from starting a fire and does not prevent gases like methane, propane and ethane from reaching the Wheatstone bridge. However, hydrocarbons like gasoline, diesel, solvents, etc, diffuse through the flame arrestor slower so that less vapor reaches the Wheatstone bridge and the sensor gives less output.
Why Not Use an LEL Monitor?
Many Volatile Organic Compounds (VOCs) are flammable and may be detected by the LEL or combustible gas sensors found in virtually every multigas monitor. However, LEL sensors are not particularly useful in measuring toxicity because they do not have enough sensitivity.
What are Some common VOC?
VOCs are the chemical compounds that keep industry going and include..
- Fuels
- Oils, °reasers, Heat Transfer Fluids
- Solvents, Paints
- Plastics, Resins and their precursors
- and many others
VOCs are found throughout industry, from the obvious applications in the petro-chem industry to not-so-obvious applications such as sausage manufacturing.

What is meant by PPM?
Parts per million (ppm) is a commonly used unit of concentration for small values. One part per million is one part of solute per one million parts solvent or 10-6. Parts per million and other "parts per" notations (e.g., parts per billion or parts per trillion) are dimensionless quantities with no units. Preferred methods for expressing parts per million include μV/V (microvolume per volume), μL/L (microliters per liter), mg/kg (milligram per kilogram), μmol/mol (micromole per mole), and μm/m (micrometer per meter).
The "parts per" notation is used to describe dilute solutions in chemistry and engineering, but its meaning is ambiguous and it is not part of the SI system of measurement. The reason the system is ambiguous is because the concentration depends on the original unit fraction that is used. For example, comparing one milliliter of a sample to a million milliliters is different from comparing one mole to a million moles or one gram to one million grams.
The University of Minnesota provides some other analogies that may help you visualize the scale involved with PPM.
One ppm is like..
- one inch in 16 miles
- one second in 11.5 days
- one minute in two years
- one car in bumper-to bumper traffic from Cleveland to San Francisco
Other visualization of scale involved with PPB
One PPB is like..
- adding a pinch of salt to a 10 ton bag of potato chips
- One ppb is like one sheet in a roll of toilet paper stretching from New York to London.
LEL Sensors Measure Explosivity, Not Toxicity
LEL sensors measure percent of LEL. For example, Gasoline has an LEL of 1.4%. Therefore, 100% of LEL is 14,000 ppm of gasoline, 10% of LEL is 1,400 ppm of gasoline and 1% of LEL is 140 ppm of gasoline.
140 ppm of gasoline is the lowest amount of vapor that the LEL monitor can "see." Gasoline has a TWA of 300 ppm and a STEL of 500 ppm; this does not make LEL sensors well suited for measuring gasoline vapors because they simply don't provide adequate resolution.
LEL sensors measure explosivity, not toxicity. Many VOCs are potentially toxic at levels that are well below their explosive levels and below the sensitivity of the LEL sensors.
As described above..
One of the many requirements for entering confined spaces called is the measurement of confined spaces for flammable gases.
Prior to entry of a confined space, the level of flammable gases must be below 10% of LEL.
The most common sensor used for measuring LEL is the Wheatstone bridge/catalytic bead/pellistor sensor ("Wheatstone bridge").
While useful in a wide variety of applications, in some settings Wheatstone bridge LEL sensors either don't have enough sensitivity to a particular chemical, or chemicals used in the environment can render the Wheatstone bridge sensor inoperable.
In these types of circumstances, PIDs (photoionization detectors) can provide an alternative, highly accurate, and poison-free means of measuring 10% of LEL for confined space entry.

What is a PID?
A Photo-Ionization Detector measures VOCs and other toxic gases in low concentrations from ppb (parts per billion) up to 10,000 ppm (parts per million or 1% by volume).
A PID is a very sensitive broad-spectrum monitor, like a "low-level LEL monitor. A Photo-Ionization Detector measures VOCs and other toxic gases in low concentrations from ppb (parts per billion) up to 10,000 ppm (parts per million or 1% by volume).
How does a PID Work?
A Photo Ionization Detector (PID) uses an Ultraviolet (UV) light source (Photo= light) to break down chemicals to positive and negative ions (Ionization) that can easily be counted with a Detector. Ionization occurs when a molecule absorbs the high energy UV light, which excites the molecule and results in the temporary loss of a negatively charged electron and the formation of positively charged ion.
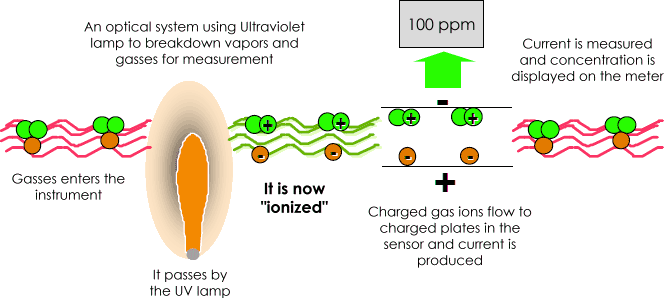
The gas becomes electrically charged. In the Detector these charged particles produce a current that is then amplified and displayed on the meter as "ppm" (parts per million) or even in "ppb" (parts per billion).
The ions quickly recombine after the electrodes in the detector to "reform" their original molecule.
PIDs are non-destructive; they do not "burn" or permanently alter the sample gas, which allows them to be used for sample gathering.
What does a PID measure?
The largest group of compounds measured by a PID are the Organics.. compounds containing Carbon (C) atoms. These include..

- Aromatics - compounds containing a benzene ring including benzene, toluene, ethyl benzene and xylene
- Ketones and Aldehydes - compounds with a C=O bond including acetone, methyl ethyl ketone (MEK) and acetaldehyde
- Amines and Amides - Carbon compounds containing nitrogen, like diethylamine
- Chlorinated hydrocarbons - trichloroethylene (TCE), perchloroethylene (PERC)
- Sulfur compounds - mercaptans, sulfides
- Unsaturated hydrocarbons - like butadiene and isobutylene
- Alcohol's- like isopropanol (IPA) and ethanol
- Saturated hydrocarbons - like butane and octane. In addition to organic compounds, PIDs can be used to measure some Inorganics. These are compounds without carbon and include..
- Ammonia
- Semiconductor gases.. Arsine, Phosphine
- Hydrogen sulfide
- Nitric Oxide
- Bromine and Iodine
Reference(s) ..
- Data extracted from Gas Data Book, 7th edition, copyright by Matheson Gas Products, and from Bulletin 627
- Flammability Characteristics of Combustible Gases and Vapors, copyright by U.S.Department of the Interior, Bureau of Mines
Related Post(s)
Generally speaking, a confined space is a fully or partially enclosed space that is not primarily designed or intended for continuous human occupancy...