Separation
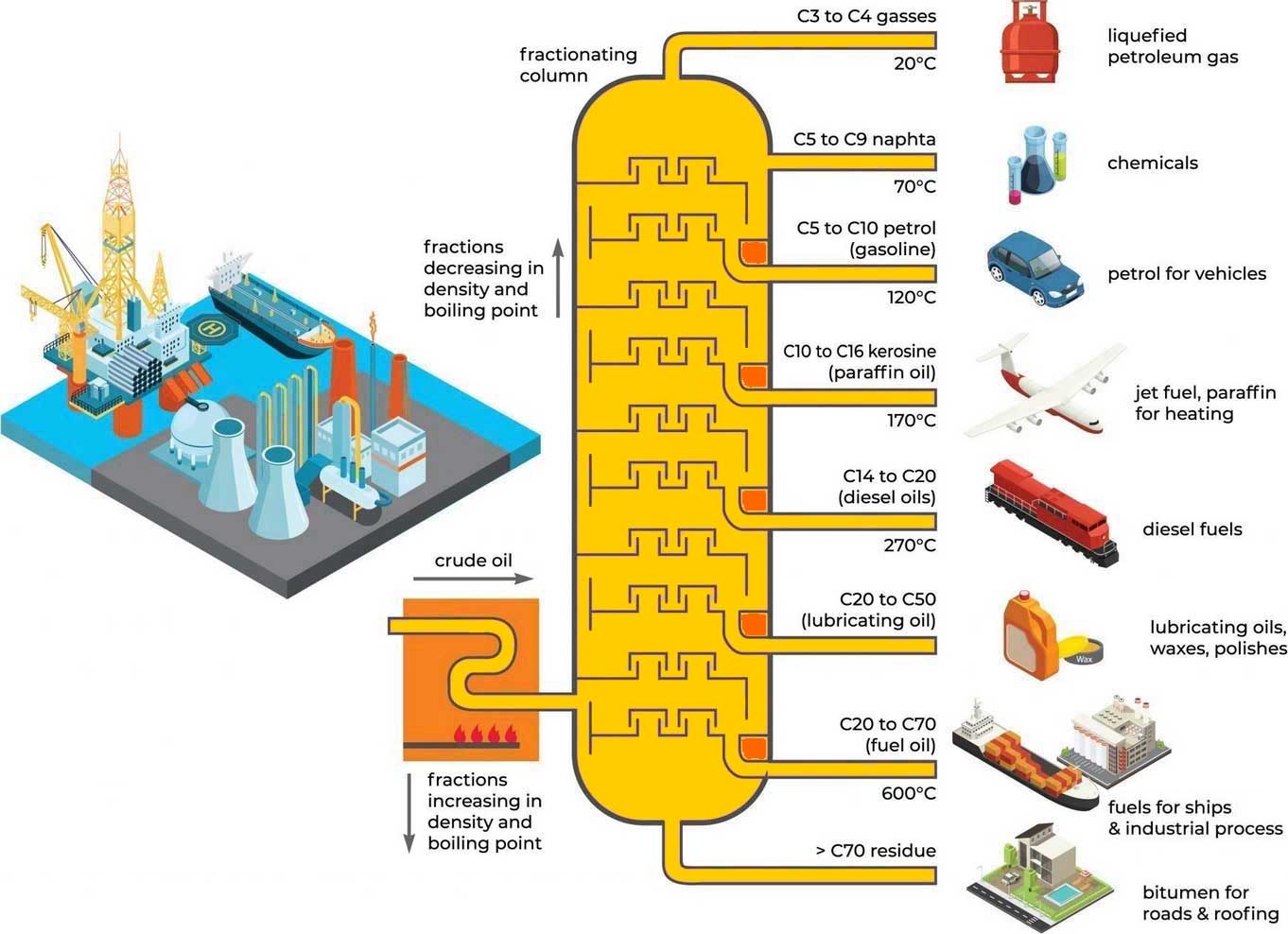
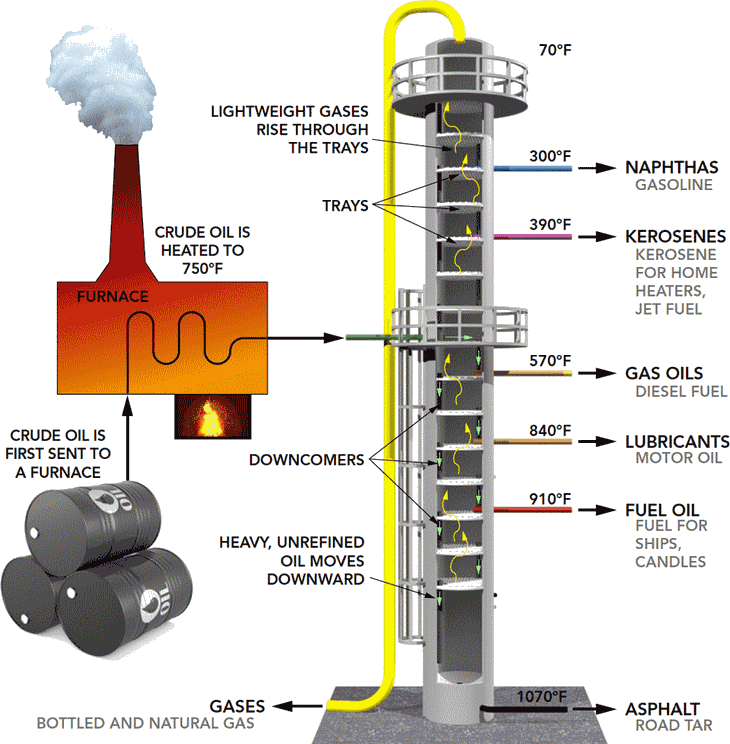
The first step is to separate the crude oil into its natural components. This is called separation
and is achieved by applying heat in a process called “distillation.”
Separation takes place in a series of distillation columns, with the bottom product of each column
being fed into the next. A furnace in front of each distillation column heats and partially vaporizes
the feed stream. The vapor-liquid mixture is then fed into the bottom of the column. The feed area
is the hottest point in the distillation tower and can reach up to 400 degrees Celsius.
Components that are still liquid at this elevated temperature become the bottom product of the
tower. Components that are in vapor form rise up the tower through a series of distillation stages.
The temperature decreases as the vapors rise up the tower and the components condense.
The “yield” of a distillation column refers to the relative percentage of each of the separated
components, known as product streams. These vary depending on the properties of the crude oil being
processed . Since the boiling point of a liquid decreases at lower pressure, the final distillation
steps under vacuum to maximize liquid recovery. The products from the distillation column range
from gases at the top to very heavy, viscous liquids at the bottom. In all cases, these product
streams are still considered “unfinished” and must be further processed to become usable products.
Conversion
Distillation breaks down crude oil into intermediate products. However, these products are not
found in crude oil in the same proportions as they are demanded by consumers. The biggest difference
is that crude oil naturally contains too little gasoline and too much heavy oil. This is why conversion
processes are so important. Their main purpose is to convert low-value heavy oil into high-value
gasoline .
All products in the refinery are based on the same building blocks, carbon and hydrogen chains,
which are called hydrocarbons. The longer the carbon chain, the heavier the product. The conversion
of heavier hydrocarbons into lighter hydrocarbons can be compared to cutting a link in a steel chain
to obtain two smaller chains. This is the function of fluidized catalytic crackers (FCCs), cokers,
and hydrocrackers. In addition to breaking chains, there are cases where we want to change the shape
of the chain or connect chains together. This is where catalytic reformers and alkylators come into
play . Special catalysts are essential for most of these processes.
The FCC is usually the most important conversion unit. It uses a catalyst (a material that helps
to accelerate a chemical reaction, allow it to take place at a lower temperature, or control which
reactions take place) to convert gas oil into a mixture of liquefied petroleum gas (LPG), gasoline,
and diesel. The FCC catalyst promotes the reaction that breaks down the heavier chains in the right
place to obtain as much gasoline as possible. However, even with the catalyst, the reactions themselves
require a lot of heat, which is why the FCC reactor is operated at around 530 degrees Celsius.
The heaviest material in the refinery is vacuum tower bottoms (VTB) or “resid.” If it cooled to
room temperature, it would become solid. In Australia, resid is sold on the market for road asphalt
or used as an additive in heating oil. Resid is too heavy and contains too many impurities to be
processed in the FCC. With a delayed coker, this heavy material can be converted into more valuable
products. The delayed coker uses high temperatures to break down the hydrocarbon chains. Delayed
coking reactions are less selective than FCC reactions. Delayed coking also produces a relatively
low-value by-product called petroleum coke.
In some refineries, FCC and delayed coker units are supplemented by hydrocracking. Similar to
FCC, hydrocracking uses high temperatures and catalysts to achieve the desired reactions. In hydrocracking,
the catalyst remains in one place and the gas oil flows over the catalyst, whereas in FCC, the catalyst
is much finer and moves along with the gas oil. The catalyst compositions differ. In hydrocracking,
the reactions take place at high temperatures in the presence of high concentrations of hydrogen.
The hydrocracker produces low-sulfur products. The light liquid product can be sent directly to
catalytic reforming, and the other liquid products can be blended directly into jet fuel and diesel.
The conversion processes discussed so far have focused on shortening the length of some hydrocarbon
chains. However, there are also other hydrocarbon chains that are too short. Butane is produced
as a by-product of other conversion units. The alkylation unit (Alky) takes two butanes and combines
them into a longer chain using a catalyst.
The final conversion process to be discussed is catalytic reforming. The purpose of the reformer
is to increase the octane rating of the gasoline blend components and to produce hydrogen for use
in the refinery's hydrotreaters. Carbon chains of equal length can have very different octane ratings
depending on the shape of the chain . Straight-chain hydrocarbons, known as paraffins, have a relatively
low octane rating, while ring-shaped hydrocarbons, also called aromatics, have high octane ratings.
At high temperatures and in the presence of hydrogen, the catalyst “reforms” paraffins into aromatics,
hence the name catalytic reforming. Some of the aromatics produced are supplied to petrochemical
manufacturers, where they are processed into plastics and textiles.
Purification
After the crude oil has been separated and converted, the resulting products are ready for purification,
which mainly involves the removal of sulfur. This is done by “hydrotreating,” a process similar
to hydrocracking, but without converting heavy molecules into lighter ones. In the hydrotreating
process , the unfinished products are brought into contact with hydrogen under heat and high pressure
in the presence of a catalyst , producing hydrogen sulfide and a desulfurized product. The catalyst
accelerates the speed at which the sulfur removal reaction takes place. In any case, sulfur removal
is essential to meet product quality specifications and environmental standards. Other units in
the refinery remove sulfur, mainly in the form of hydrogen sulfide, by extraction, a second purification
method.
Regardless of whether it is done by hydrotreating or extraction, hydrogen sulfide is produced
during desulfurization. During sulfur recovery, hydrogen sulfide is converted into elemental sulfur
and water. The remaining sulfur is sold as a by-product of the refinery.
End products
Modern refining and petrochemical technology can convert crude oil into literally thousands of useful
products. From powering our cars and heating our homes to supplying petrochemical feedstocks for
the manufacture of plastics and medicines, crude oil is an essential part of our daily lives. It
is an important ingredient in the manufacture of thousands of products that make our lives easier
– and in many cases – help us to live better and longer.

Oil does much more than just provide fuel for our cars and trucks, keep our homes and offices
comfortable, and power our industries. From lipstick to aspirin to rollerblades, petrochemicals
play an important role. Here are some examples of products made from petrochemicals.
- Antiseptics
- Golf Balls
- Aspirin
- House Paint
- Baby Strollers
- Jet Fuel
- Balloons
- Medical
- Equipment
- Cameras
- Motor Oil
- Perfumes
- CD Players
- Photographs
- Clothing
- Roller Blades
- Compact Discs
- Shampoo
- Deodorant
- Sunglasses
- Disposable Nappies
- Telephones
- DVDs
- Tyres
- Toothpaste
- Petrol
- Toys
- Garbage Bags
- Umbrellas
- Glue
- Vitamin Capsules
Related Post(s)
Distillation Column
There are many types of distillation columns, each designed to perform specific types of separations,
and each design differs in terms of complexity...
Most Popular Pages
Popular on wermac.org - Butt Weld Elbows -%LEL / %UEL / PPM / PID - Spectacle blinds - Pipe Flanges
General - Dimensions Spiral Wound Gaskets etc.
updated per month